About Sodium Thiosulfate
All products are for external use only
Free shipping notes: 40 Lb Bag in Box
40 Lb Bag in box ships free in lower 48 continental states. Soapgoods Inc reserves the right to ship free shipping items via any carrier.
Documentation
Identification
- Synonyms: Sodium Thiosulfate, Sodium Thiosulfate Pentahydrate
- INCI Name: Sodium Thiosulfate
- CAS: 7772-98-7
- Einecs: 231-867-5
The Science
- Viscosity: Solid
- Solublity: Water soluble
- Storage: Cool, dark dry area, air tight container preferred
Characteristics
- Appearance: White and clear crystal like pieces
- Odor: ??
- Natural: Synthetic
- Packaging: 1 Lbs is a single plastic bag, 4 Lb is a single plastic bag, 55 lb is single bag
- Shelf life: Suggested retest date 1 year from purchase
Usage / Benefits
- Industries: Photography, medical, textile, water treatment, paper, metal extraction and refining, chemical manufacturing, tanning, agriculture, food processing, environmental monitoring
- Applications: Photographic fixer, antidote for cyanide poisoning, chlorine neutralizer in water treatment, iodometric titrations, gold extraction, leather tanning, dechlorination in aquaculture, antifungal treatment in crops, bleach neutralizer in paper industry, eczema treatment in medical applications, reducing agent in chemical synthesis
- Benefits: Versatile reducing agent, effective antidote for cyanide poisoning, useful in gold extraction, neutralizes chlorine in water treatment, promotes leather softening in tanning, environmentally friendly, helps in treating certain medical conditions, essential in photographic processing, aids in analytical chemistry, can be used as a fungicide in agriculture.
- Products Uses: Photographic fixer in photography, antidote in cyanide poisoning treatment, chlorine neutralizer in water treatment plants, reagent in iodometric titrations, agent in gold and silver extraction, softening treatment in leather tanning, bleach neutralizer in paper industry, treatment for certain skin conditions, reducing agent in various chemical processes, fungicide in agricultural applications.
- Safety: Generally safe, however keep out of eyes and do not eat.
- Cautions: Not for ingestion, keep away from pets and children who may attempt to eat.
- External Use Only: Even if food grade, we do not provide items for ingestion, all of our items are for external use only.
Sodium Thiosulfate
Sodium thiosulfate, commonly known as "hypo", is a versatile and valuable chemical compound with a myriad of applications. When considering the realms of personal care, soap making, and cosmetics, sodium thiosulfate emerges as a useful and often integral ingredient, offering various functionalities to enhance product performance and user experience. This article delves deep into the world of sodium thiosulfate, highlighting its characteristics, benefits, and specific uses within the cosmetic and personal care sectors.
What is Sodium Thiosulfate?
Sodium thiosulfate is an inorganic salt, crystalline in nature, and is highly soluble in water. Its chemical formula is Na2S2O3. Traditionally used in the photographic industry as a fixing agent, its role in personal care and cosmetic formulations is relatively less known but no less significant.
Characteristics of Sodium Thiosulfate
The unique physicochemical properties of sodium thiosulfate make it a favorable ingredient in various formulations. Its high solubility ensures easy integration into aqueous solutions. Moreover, it is generally regarded as safe and non-toxic, making it suitable for topical applications. Being neutral in pH, it's often considered non-irritating and gentle on the skin.
Role in Personal Care Products
Within the personal care domain, sodium thiosulfate finds its presence in a number of applications:
- Antioxidant Properties: Sodium thiosulfate acts as a potent antioxidant. In cosmetic formulations, it can protect products from oxidative degradation, ensuring they maintain their efficacy and shelf life.
- Detoxifying Agent: Owing to its capability to neutralize chlorine, sodium thiosulfate is often included in shower gels and bath preparations, especially those meant for swimmers or those exposed to chlorinated water. It aids in removing residual chlorine from the skin and hair, preventing dryness and irritation.
- Stabilizer: In certain formulations, sodium thiosulfate can act as a stabilizing agent, ensuring that the product retains its consistency, texture, and quality over time.
Contribution to Soap Making
For soap makers, sodium thiosulfate offers several advantages:
- Chelating Agent: In hard water conditions, certain metal ions can hinder the lathering capability of soaps. Sodium thiosulfate acts as a chelating agent, binding to these ions and enhancing the foaming property of soaps.
- Enhancing Soap Clarity: In the production of transparent or clear soaps, sodium thiosulfate can play a role in improving clarity by removing any residual impurities.
Presence in Cosmetic Formulations
Sodium thiosulfate's inclusion in cosmetic preparations enhances their performance in several ways:
- Skin Protection: The antioxidant properties of sodium thiosulfate can shield the skin from environmental stressors, preventing premature aging.
- Enhancing Product Stability: As a stabilizer and antioxidant, sodium thiosulfate ensures that cosmetic products, especially those containing sensitive ingredients, remain stable and effective during their shelf life.
Sodium Thiosulfate Uses
Photography
Utilized as a photographic fixer, essential in the development of traditional photographs to stabilize the image.
Medical Applications
Administered as an antidote for cyanide poisoning and used in the treatment of certain skin conditions like eczema.
Water Treatment
Employed to neutralize chlorine in water treatment facilities, protecting aquatic life and enhancing water quality.
Metal Extraction
Used in the extraction process for metals such as gold and silver, facilitating the recovery of precious metals.
Textile and Leather Tanning
Applied in the softening treatment of leather and as a bleaching agent in textiles, improving quality and appearance.
Paper Industry
Acting as a bleach neutralizer, it plays a role in the production and processing of paper products.
Chemical Synthesis
Employed as a versatile reducing agent in various chemical synthesis and analytical procedures, such as iodometric titrations.
Agriculture
Used as a fungicide, Sodium Thiosulfate helps in controlling fungal diseases in crops, contributing to better yield and plant health.
Environmental Considerations
Recognized for its environmentally friendly properties, it is an essential component in environmental monitoring and protection efforts.
Sodium Thiosulfate Benefits
Sodium thiosulfate, commonly known as "hypo", has a myriad of benefits that make it a versatile and indispensable compound in various applications. In the context of personal care, soap making, and cosmetics, these benefits are particularly pronounced. Below are the key advantages of incorporating sodium thiosulfate into such formulations.
1. Antioxidant Properties
Sodium thiosulfate possesses strong antioxidant capabilities. This means it can protect cosmetic and personal care products from oxidative degradation, ensuring they maintain their efficacy and have an extended shelf life. Furthermore, its antioxidant properties can shield the skin from environmental stressors, potentially preventing premature aging.
2. Detoxifying Agent
Known for its ability to neutralize chlorine, sodium thiosulfate is often found in formulations meant for those exposed to chlorinated water, such as swimmers. It efficiently removes residual chlorine from skin and hair, preventing the associated dryness, irritation, and potential damage.
3. Stabilizer in Formulations
Within various cosmetic and personal care products, sodium thiosulfate can act as a stabilizing agent. It ensures that the product maintains a consistent texture, quality, and performance throughout its shelf life.
4. Chelating Properties
In hard water conditions, certain metal ions can inhibit the optimal performance of soaps and detergents. As a chelating agent, sodium thiosulfate binds to these ions, enhancing the efficacy and foaming capacity of soap products, and can also contribute to the clarity of transparent soaps by removing residual impurities.
5. Safety and Non-Toxicity
One of the significant benefits of sodium thiosulfate is its safety profile. It's generally regarded as non-toxic and non-irritating, making it suitable for topical applications in cosmetics and personal care products, even for those with sensitive skin.
FAQ
Sodium Thiosulfate Pentahydrate: Chemical Formula
Sodium Thiosulfate Pentahydrate is a chemical compound with a specific chemical formula:
1. Chemical Composition
The chemical formula for Sodium Thiosulfate Pentahydrate is Na2S2O3 • 5H2O. This formula represents the arrangement of atoms in the compound.
2. Components
The formula can be broken down into its components:
Sodium (Na): The sodium cations (Na+) contribute to the compound's overall charge.
Thiosulfate (S2O32-): Thiosulfate anions are composed of sulfur and oxygen atoms and carry a negative charge.
Pentahydrate (5H2O): The "Pentahydrate" indicates that the compound is associated with five water molecules (H2O), forming a hydrated crystal structure.
3. Structure
Sodium Thiosulfate Pentahydrate's chemical formula describes its atomic arrangement and the presence of water molecules within its crystal lattice.
4. Applications
The compound's formula influences its properties and applications in various fields, including photography, medicine, and water treatment.
Understanding the Formula
The chemical formula of Sodium Thiosulfate Pentahydrate, Na2S2O3 • 5H2O, provides insight into its composition, structure, and potential applications across different industries.
Sodium Thiosulfate Pentahydrate vs. Sodium Thiosulfate Anhydrous
Sodium Thiosulfate Pentahydrate and Sodium Thiosulfate Anhydrous are two forms of the same compound with distinct characteristics and applications:
1. Hydration State
Sodium Thiosulfate Pentahydrate: In this form, the compound is associated with five water molecules (H2O), making it a hydrated crystal.
Sodium Thiosulfate Anhydrous: In this form, the compound is devoid of water molecules, meaning it exists in a completely dry state.
2. Chemical Formula
Sodium Thiosulfate Pentahydrate: The chemical formula is Na2S2O3 • 5H2O, indicating the presence of both the thiosulfate anions and five water molecules.
Sodium Thiosulfate Anhydrous: The chemical formula is Na2S2O3, representing the compound without any associated water molecules.
3. Properties
Sodium Thiosulfate Pentahydrate: The presence of water molecules affects its physical and chemical properties, such as solubility and crystal structure.
Sodium Thiosulfate Anhydrous: Being in a dry state influences its properties and behavior in various applications.
4. Applications
Sodium Thiosulfate Pentahydrate: It's commonly used in applications where controlled water release is desired, such as in photographic fixer solutions.
Sodium Thiosulfate Anhydrous: It finds applications in processes where the absence of water is critical, such as certain chemical reactions and water treatment.
Choosing the Appropriate Form
When choosing between Sodium Thiosulfate Pentahydrate and Sodium Thiosulfate Anhydrous:
1. Application Requirements
Consider the role of water in your application. If controlled water content is needed, the pentahydrate form might be preferred.
2. Reaction Conditions
For specific reactions or processes, the presence or absence of water may influence the outcome.
Both forms of Sodium Thiosulfate serve different purposes based on their hydration states, influencing their properties and suitability for various applications.
Sodium Thiosulfate Pentahydrate Molar Mass
The molar mass of Sodium Thiosulfate Pentahydrate is a key value used in chemical calculations:
1. Chemical Composition
The chemical formula for Sodium Thiosulfate Pentahydrate is Na2S2O3 • 5H2O. This indicates the arrangement of atoms and water molecules in the compound.
2. Molar Mass Calculation
The molar mass is the sum of the atomic masses of all the elements present in the compound:
Sodium (Na): Atomic mass ≈ 22.99 g/mol
Sulfur (S): Atomic mass ≈ 32.07 g/mol
Oxygen (O): Atomic mass ≈ 16.00 g/mol
Hydrogen (H): Atomic mass ≈ 1.01 g/mol
Since there are five water molecules, the contribution of water is multiplied by 5.
3. Molar Mass Calculation
The molar mass can be calculated as follows:
Molar mass of Na2S2O3 • 5H2O = (2 * Na) + (2 * S) + (3 * O) + (5 * H2O)
Molar mass ≈ (2 * 22.99 g/mol) + (2 * 32.07 g/mol) + (3 * 16.00 g/mol) + (5 * 18.02 g/mol)
4. Calculated Molar Mass
The calculated molar mass of Sodium Thiosulfate Pentahydrate is approximately 248.18 g/mol.
Significance of Molar Mass
The molar mass of Sodium Thiosulfate Pentahydrate is a crucial value for determining the quantity of substance in chemical reactions, formulation, and various applications.
Who is Soapgoods?
We are proud to present a diverse and extensive selection of soap making supplies including soap molds as well as melt and pour soap bases. Are you looking for something unique, something hard to find? Wondering Where to buy Witch Hazel Distillate - Alcohol Free? We are a fantastic source!
We carry it all and many other fantastic but hard to find items at great wholesale prices, we are the one-stop-shop, you can find everything you need here, even bulk Xanthan Gum Right here at Soapgoods
We know you have choices, that's why we work harder, providing better quality, faster shipping and a wide selection of items in practical sizes for any application. When you need to make a purchase and need it delivered quickly, look to us to provide you the service you need. We even have Wholesale Yogurt Powder, in small and large builds sizes, either way, you can get our best direct to consumer pricing. Plus we ship it out the next business days. and will ship the next business day.
Where else can you do all your shopping in one place? When you need to make a purchase and get it delivered quickly. Buy Zinc Oxide Powder? Right here
When you are looking for quality personal care products, cosmetics and soap making supplies, be sure to visit our online store for Acacia Gum Enjoy our premium quality ingredients and super fast shipping times.
Read our Real Reviews from Google and Bizrate
100% Real Reviews (we cannot edit, create or delete reviews) From Google, Click here -> Real SoapGoods Reviews
See more Real Reviews (we cannot edit, create or delete reviews) from Third Party Watchdog Bizrate Real SoapGoods Reviews (Click Here and Scroll Down)
Read hundreds of real testimonials from over a decade of service. SoapGoods Testimonials.
How Fast Can I get it?
We Guarantee Your order ships out the same or next business day! This means in the South East you will have your order in 1 to 3 business days, in the North East normally 3 to 4 days and in the West normally 4 to 5 days. For full details on shipping and processing times please see our expected delivery times.
These times are based on business days, not including weekends or holidays.
FedEx Delivery Map
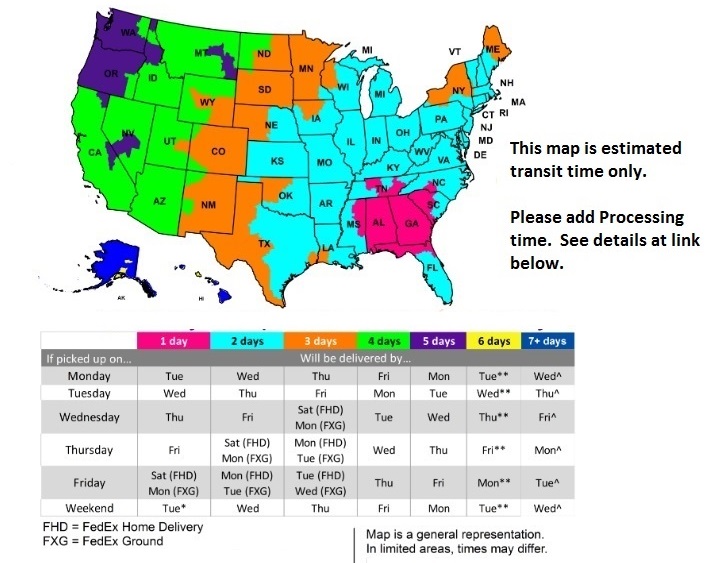
For Processing Times Click Here
USPS Delivery Map
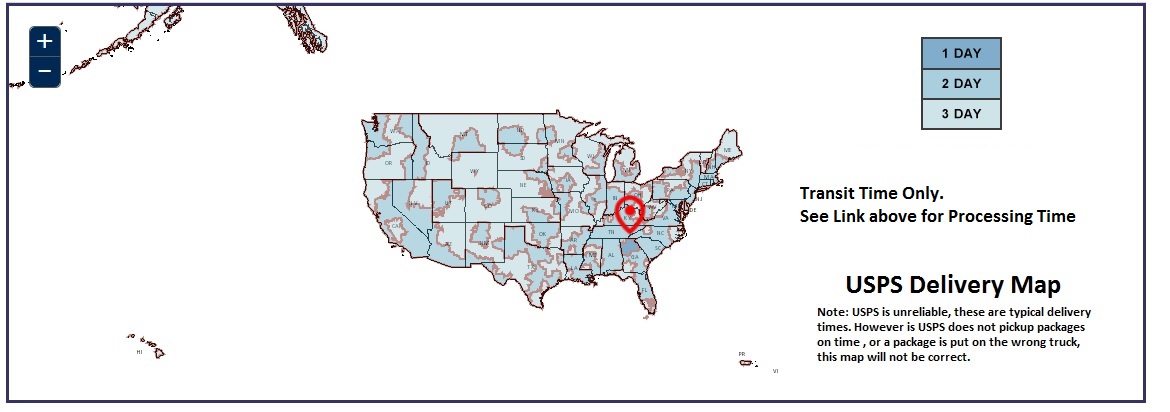
Typical Delivery Times to Major US Cities
| Major Cities | Total Business Days +1 / -1 |
|---|---|
| Alabama (AL) - Montgomery, Birmingham | 1 |
| Alaska (AK) - Juneau, Anchorage | 7 |
| Arizona (AZ) - Phoenix, Tucson | 4 |
| Arkansas (AR) - Little Rock, Fayetteville | 2 |
| California (CA) - Sacramento, Los Angeles, San Francisco, San Diego, Sacramento, San Jose | 4 |
| Colorado (CO) - Denver, Colorado Springs | 3 |
| Connecticut (CT) - Hartford, New Haven | 2 |
| Delaware (DE) - Dover, Wilmington, Newark | 2 |
| Florida (FL) - Tallahassee, Orlando, Miami, Jacksonville, Tampa, Destin | 2 |
| Georgia (GA) - Atlanta, Savannah, Augusta, Athens | 1 |
| Hawaii (HI) - Honolulu, Kailua | 7 |
| Idaho (ID) - Boise, Coeur d'Alene | 4 |
| Illinois (IL) - Springfield, Chicago, Peoria, Rockford | 2 |
| Indiana (IN) - Indianapolis, Fort Wayne | 2 |
| Iowa (IA) - Des Moines, Cedar Rapids | 2 |
| Kansas (KS) - Topeka, Wichita, Kansas City | 2 |
| Kentucky (KY) - Frankfort, Louisville, Lexington | 2 |
| Louisiana (LA) - Baton Rouge, New Orleans, Lafayette | 2 |
| Maine (ME) - Augusta, Portland, Bangor | 3 |
| Maryland (MD) - Annapolis, Baltimore | 2 |
| Massachusetts (MA) - Boston, Cambridge, Worcester | 2 |
| Michigan (MI) - Lansing, Detroit, Grand Rapids | 2 |
| Minnesota (MN) - St. Paul, Minneapolis, Duluth | 3 |
| Mississippi (MS) - Jackson, Biloxi, Hattiesburg | 1 |
| Missouri (MO) - Jefferson City, St Louis, Kansas City | 2 |
| Montana (MT) - Helena, Billings | 4 |
| Nebraska (NE) - Lincoln, Omaha | 2 |
| Nevada (NV) - Carson City, Las Vegas, Reno | 4 |
| New Hampshire (NH) - Concord, Manchester, Portsmouth | 2 |
| New Jersey (NJ) - Trenton, Newark, Jersey City | 2 |
| New Mexico (NM) - Santa Fe, Alburquerque | 3 |
| New York (NY) - Albany, New York, Rochester, Buffalo, Albany, Syracuse, Niagara Falls, Ithaca | 3 |
| North Carolina (NC) - Raleigh, Charlotte | 2 |
| North Dakota (ND) - Bismarck, Fargo | 3 |
| Ohio (OH) - Columbus, Cleveland, Cincinnati | 2 |
| Oklahoma (OK) - Oklahoma City, Fairview, | 2 |
| Oregon (OR) - Salem, Portland, Eugene | 5 |
| Pennsylvania (PA) - Harrisburg, Philadelphia, Pittsburgh | 2 |
| Rhode Island (RI) - Providence, Newport | 2 |
| South Carolina (SC) - Columbia, Charleston | 1 |
| South Dakota (SD) - Pierre, Sioux Falls, Rapid City | 3 |
| Tennessee (TN) - Nashville, Memphis | 2 |
| Texas (TX) - Austin, Houston, Dallas | 3 |
| Utah (UT) - Salt Lake City, St. George | 3 |
| Vermont (VT) - Montpelier, Burlington | 3 |
| Virginia (VA) - Richmond, Virginia Beach | 2 |
| Washington (WA) - Olympia, Seattle, Vancouver, Spokane | 5 |
| West Virginia (WV) - Charleston, Morgantown | 2 |
| Wisconsin (WI) - Madison, Milwaukee | 2 |
| Wyoming (WY) - Cheyenne, Jackson | 4 |
Disclaimer: All product descriptions and specifications provided in this description are intended as a guide only and are subject to change without notice. While we strive for accuracy, discrepancies or errors may be present. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
No posts found











