About Sodium Acetate
All products are for external use only
Free shipping notes: 40 Lb Bag in Box
40 Lb Bag in box ships free in lower 48 continental states. Soapgoods Inc reserves the right to ship free shipping items via any carrier.
Documentation
Identification
- Synonyms: Sodium Acetate, Sodium Acetate Anhydrous
- INCI Name: Sodium Acetate
- CAS: 127-09-3
- Einecs: 204-823-8
The Science
- Grade: Technical Grade
- Viscosity: Dry Powder
- Solublity: Soluble in water
- Storage: Cool, dark dry area, air tight container preferred
Characteristics
- Appearance: White Powder
- Odor: Medium characteristic scent
- Natural: Synthetically derived from natural product.
- Packaging: 1 Lbs is a single bag, 5 Lb is a single bag, 50 lb is a single 50 lb bag. 500 lb is 10 x 50 lb bag
- Shelf life: Suggested retest date 1 year from purchase
Usage / Benefits
- Industries: Photography, textiles, food preservation, laboratory, heating pads, leather tanning, wastewater treatment, concrete longevity, electroplating, cosmetics, personal care products
- Applications: Buffering agent, laboratory reagent, mordant in dyeing processes, pH regulator, heating pad component, flavor enhancer, preservative in snack foods, tanning agent in leather, enhancer for concrete longevity, electroplating bath component, moisture retention agent in cosmetics.
- Benefits: Stabilizes pH levels, safe for most applications, enhances flavors, extends shelf life of certain foods, boosts concrete durability, aids in efficient metal deposition, promotes even dye uptake, provides controlled exothermic reactions in heating pads, moisturizes in cosmetic formulations.
- Products Uses: pH regulation in cosmetics, flavoring agent in food, concrete sealant additive, electroplating bath component, textile dyeing aid, heating pad ingredient, buffer in laboratory applications, moisturizing agent in skin care products, preservative in some food items.
- Safety: Generally safe, however keep out of eyes and do not eat.
- Cautions: Not for ingestion, keep away from pets and children who may attempt to eat.
- External Use Only: Even if food grade, we do not provide items for ingestion, all of our items are for external use only.
Sodium Acetate
Sodium acetate, chemically represented as NaC2H3O2, is the sodium salt of acetic acid. It is a white crystalline powder which, when dissolved in water, dissociates into sodium ions and acetate ions. Beyond its uses in food and pharmaceuticals, sodium acetate has found applications in the personal care and cosmetic industry due to its unique chemical properties.
Role in Personal Care Products
In the realm of personal care products, sodium acetate is often employed for its capacity to adjust the pH levels of various formulations. Maintaining a product's pH at the optimal level is crucial for ensuring the efficacy and stability of the product. Sodium acetate acts as a buffer, helping to stabilize the pH, especially in hair care and skincare products.
Moisturizing Properties
While not a primary moisturizing agent, sodium acetate plays a role in retaining moisture in cosmetic formulations. It can work in tandem with other moisturizing ingredients to help maintain skin hydration, thus keeping the skin feeling soft and refreshed.
Soap Making
In soap making, sodium acetate is used as a secondary ingredient. One of its main benefits in this context is to help in the saponification process. By adjusting the pH level, it aids in the smooth formation of soap, ensuring a consistent and desirable texture. Furthermore, its buffering capacity can help in maintaining the pH of the finished soap, ensuring its long-term stability and skin-compatibility.
Cosmetic Making
The cosmetic industry values ingredients that can bring stability to its products, and sodium acetate fits the bill. Its ability to stabilize pH is crucial in ensuring that cosmetics maintain their intended texture, efficacy, and shelf life.
As a Preservative Enhancer
In some cosmetic formulations, sodium acetate is used in conjunction with other preservatives to enhance their efficiency. By adjusting the pH of the formulation, sodium acetate can make the environment less hospitable for microbial growth, thereby extending the product's shelf life.
Challenges and Considerations
While sodium acetate offers many benefits, it's essential to note that its use in personal care and cosmetics should be in recommended concentrations to ensure safety and product efficacy. Overuse might lead to skin irritations in sensitive individuals or could impact the overall texture and feel of the final product.
Sodium Acetate Uses
Sodium Acetate is used in various industrial applications including:
- Textile Industry: as a dye fixative
- Leather Industry: for tanning and re-tanning processes
- Photographic Industry: as a buffering agent
Food Industry
As a Preservative
Sodium Acetate is often used as a food preservative due to its ability to inhibit microbial growth.
As a Flavor Enhancer
It's also used as a seasoning in certain food products, giving a salty and vinegar-like flavor.
Medical Uses
In the medical field, Sodium Acetate is used in various ways such as:
- As an electrolyte solution in intravenous (IV) fluids
- In heating pads: as a reusable heat source
Environmental Uses
It has been used in waste water treatment processes to neutralize sulfuric acid waste and other industrial chemicals.
Sodium Acetate Benefits
Rehydration:
Used in intravenous solutions to correct electrolyte imbalances and provide rehydration.Heat Generation:
Found in reusable heating pads to provide controlled release of heat.
Industrial Benefits
Dye Fixative:
Acts as a mordant in dyeing processes, enhancing color retention.Neutralizing Agent:
Used to neutralize industrial waste products and control pH levels.
Food Industry Benefits
Preservation:
Effective in preserving food by inhibiting microbial growth.Flavor Enhancement:
Enhances flavor in certain food products, imparting a salty, vinegar-like taste.
Environmental Benefits
Waste Water Treatment:
Contributes to environmental protection by treating waste water and neutralizing harmful chemicals.
FAQ
Sodium Acetate - Anhydrous Formula
Sodium Acetate - Anhydrous is a chemical compound with a specific formula reflecting its composition:
1. Chemical Composition
The chemical formula for Sodium Acetate - Anhydrous is CH3COONa.
2. Molecular Structure
Sodium Acetate - Anhydrous consists of carbon atoms (C), hydrogen atoms (H), sodium atoms (Na), and oxygen atoms (O). Its molecular structure includes an acetate group.
3. Anhydrous Nature
The term "anhydrous" indicates that Sodium Acetate is in its dry, crystalline form, meaning it does not contain any water molecules within its structure.
Applications of Sodium Acetate - Anhydrous
Sodium Acetate - Anhydrous is utilized in various industries for its distinct properties beyond its potential uses in food-related contexts.
Who is Soapgoods?
We are proud to present a diverse and extensive selection of soap making supplies including soap molds as well as melt and pour soap bases. Are you looking for something unique, something hard to find? Wondering Where to buy Witch Hazel Distillate - Alcohol Free? We are a fantastic source!
We carry it all and many other fantastic but hard to find items at great wholesale prices, we are the one-stop-shop, you can find everything you need here, even bulk Xanthan Gum Right here at Soapgoods
We know you have choices, that's why we work harder, providing better quality, faster shipping and a wide selection of items in practical sizes for any application. When you need to make a purchase and need it delivered quickly, look to us to provide you the service you need. We even have Wholesale Yogurt Powder, in small and large builds sizes, either way, you can get our best direct to consumer pricing. Plus we ship it out the next business days. and will ship the next business day.
Where else can you do all your shopping in one place? When you need to make a purchase and get it delivered quickly. Buy Zinc Oxide Powder? Right here
When you are looking for quality personal care products, cosmetics and soap making supplies, be sure to visit our online store for Acacia Gum Enjoy our premium quality ingredients and super fast shipping times.
Read our Real Reviews from Google and Bizrate
100% Real Reviews (we cannot edit, create or delete reviews) From Google, Click here -> Real SoapGoods Reviews
See more Real Reviews (we cannot edit, create or delete reviews) from Third Party Watchdog Bizrate Real SoapGoods Reviews (Click Here and Scroll Down)
Read hundreds of real testimonials from over a decade of service. SoapGoods Testimonials.
How Fast Can I get it?
We Guarantee Your order ships out the same or next business day! This means in the South East you will have your order in 1 to 3 business days, in the North East normally 3 to 4 days and in the West normally 4 to 5 days. For full details on shipping and processing times please see our expected delivery times.
These times are based on business days, not including weekends or holidays.
FedEx Delivery Map
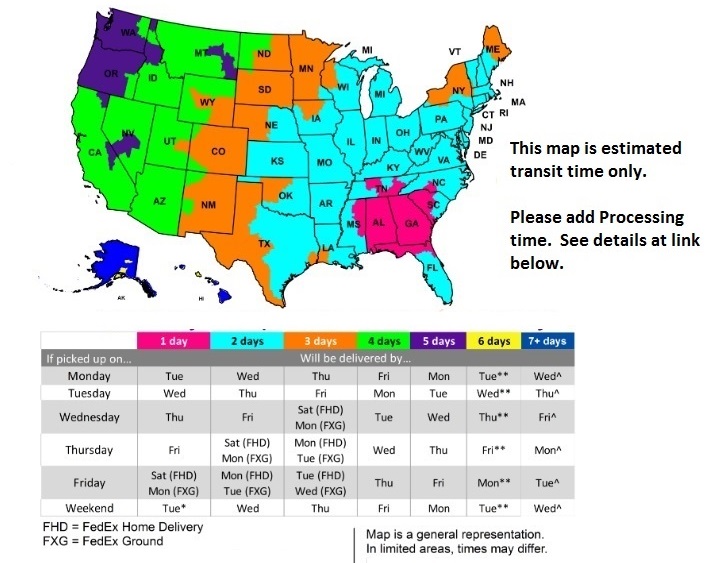
For Processing Times Click Here
USPS Delivery Map
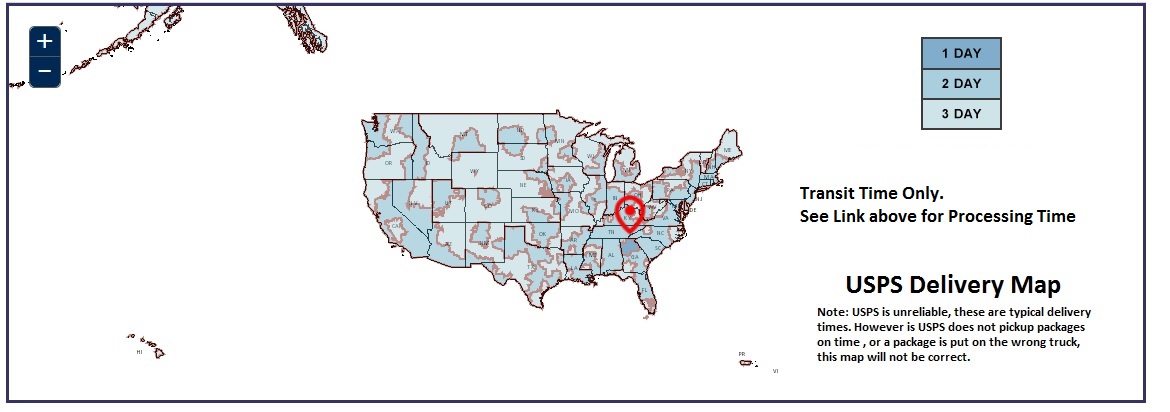
Typical Delivery Times to Major US Cities
| Major Cities | Total Business Days +1 / -1 |
|---|---|
| Alabama (AL) - Montgomery, Birmingham | 1 |
| Alaska (AK) - Juneau, Anchorage | 7 |
| Arizona (AZ) - Phoenix, Tucson | 4 |
| Arkansas (AR) - Little Rock, Fayetteville | 2 |
| California (CA) - Sacramento, Los Angeles, San Francisco, San Diego, Sacramento, San Jose | 4 |
| Colorado (CO) - Denver, Colorado Springs | 3 |
| Connecticut (CT) - Hartford, New Haven | 2 |
| Delaware (DE) - Dover, Wilmington, Newark | 2 |
| Florida (FL) - Tallahassee, Orlando, Miami, Jacksonville, Tampa, Destin | 2 |
| Georgia (GA) - Atlanta, Savannah, Augusta, Athens | 1 |
| Hawaii (HI) - Honolulu, Kailua | 7 |
| Idaho (ID) - Boise, Coeur d'Alene | 4 |
| Illinois (IL) - Springfield, Chicago, Peoria, Rockford | 2 |
| Indiana (IN) - Indianapolis, Fort Wayne | 2 |
| Iowa (IA) - Des Moines, Cedar Rapids | 2 |
| Kansas (KS) - Topeka, Wichita, Kansas City | 2 |
| Kentucky (KY) - Frankfort, Louisville, Lexington | 2 |
| Louisiana (LA) - Baton Rouge, New Orleans, Lafayette | 2 |
| Maine (ME) - Augusta, Portland, Bangor | 3 |
| Maryland (MD) - Annapolis, Baltimore | 2 |
| Massachusetts (MA) - Boston, Cambridge, Worcester | 2 |
| Michigan (MI) - Lansing, Detroit, Grand Rapids | 2 |
| Minnesota (MN) - St. Paul, Minneapolis, Duluth | 3 |
| Mississippi (MS) - Jackson, Biloxi, Hattiesburg | 1 |
| Missouri (MO) - Jefferson City, St Louis, Kansas City | 2 |
| Montana (MT) - Helena, Billings | 4 |
| Nebraska (NE) - Lincoln, Omaha | 2 |
| Nevada (NV) - Carson City, Las Vegas, Reno | 4 |
| New Hampshire (NH) - Concord, Manchester, Portsmouth | 2 |
| New Jersey (NJ) - Trenton, Newark, Jersey City | 2 |
| New Mexico (NM) - Santa Fe, Alburquerque | 3 |
| New York (NY) - Albany, New York, Rochester, Buffalo, Albany, Syracuse, Niagara Falls, Ithaca | 3 |
| North Carolina (NC) - Raleigh, Charlotte | 2 |
| North Dakota (ND) - Bismarck, Fargo | 3 |
| Ohio (OH) - Columbus, Cleveland, Cincinnati | 2 |
| Oklahoma (OK) - Oklahoma City, Fairview, | 2 |
| Oregon (OR) - Salem, Portland, Eugene | 5 |
| Pennsylvania (PA) - Harrisburg, Philadelphia, Pittsburgh | 2 |
| Rhode Island (RI) - Providence, Newport | 2 |
| South Carolina (SC) - Columbia, Charleston | 1 |
| South Dakota (SD) - Pierre, Sioux Falls, Rapid City | 3 |
| Tennessee (TN) - Nashville, Memphis | 2 |
| Texas (TX) - Austin, Houston, Dallas | 3 |
| Utah (UT) - Salt Lake City, St. George | 3 |
| Vermont (VT) - Montpelier, Burlington | 3 |
| Virginia (VA) - Richmond, Virginia Beach | 2 |
| Washington (WA) - Olympia, Seattle, Vancouver, Spokane | 5 |
| West Virginia (WV) - Charleston, Morgantown | 2 |
| Wisconsin (WI) - Madison, Milwaukee | 2 |
| Wyoming (WY) - Cheyenne, Jackson | 4 |
Disclaimer: All product descriptions and specifications provided in this description are intended as a guide only and are subject to change without notice. While we strive for accuracy, discrepancies or errors may be present. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
Where to Buy Sodium Acetate
Wondering where you can purchase Sodium Acetate? Let us help, we carry Sodium Acetate Anhydrous that is a Technical grade. You will want to choose a supplier that has been around a while and can provide product quickly. Soapgoods has been in business since 2006 and normally ships items out the same or next business day. We also have a 45-day satisfaction guarantee.
Where is Sodium Acetate from?
Sodium acetate is a manufactured product, manufactured industrially for consumer use. It may be unnaturally produced, however, it is made from the sodium salt of acetic acid which is an organic compound.

It is possible to make small batches of Sodium Acetate at home, you can find some ideas on making your own Sodium Acetate here
Geographically our Sodium Acetate is Made in the USA
Which Grade do you need
Sodium Acetate is available in several types and grades including Anhydrous and Trihydrate as well as pharmaceutical and technical grades. The Anhydrous is normally a powder while the Trihydrate is typically a granular crystal. Also, the Trihydrate is very soluble in water compared to the anhydrous version. The anhydrous offers different characteristics including low dustiness, higher reactivity, higher density, and improved free-flow-ability. The molecular weight of the Trihydrate is 136.08 g/mol compared to the anhydrous at 82.04 g/mol. The Cas No for Sodium Acetate Anhydrous is 127-09-03, the Trihydrate is identified by CAS No. 6131-90-4
Is Sodium Acetate Dangerous
In general Sodium Acetate is a fairly safe chemical, in fact, it is on the FDA's list of Generally Recognized as Safe (Gras) chemicals. In relatively small levels it is benign in the environment, and not considered harmful.
In fact, it is a major component of Sodium diacetate that is a very common preservative and flavor enhancer used in many foods. It is however considered to be an eye and a little bit of a skin irritant in some situations.
The other caution is that Sodium Acetate can be flammable if brought into contact with open flames, it also reacts if mixed with oxidizers such as nitrc acid or potassium nitrate.
How Long can you Keep Sodium Acetate
Sodium Acetate, if stored in ideal storage conditions can last anywhere from 2 to 4 years or more. It is best to keep it dry, cool and out of the sun, an airtight container would work great. Regular testing after the 2 years will ensure the sodium acetate still works well in your application. However if at all possible it is best to only purchase quantities that will be used in 2 years to ensure ideal performance.
How is Sodium Acetate Made
Sodium acetate is manufactured by reacting acetic acid (vinegar) with sodium hydroxide. There are other manufacturing methods including using sodium carbonate instead of the sodium hydroxide. A manufacturer could even create sodium acetate by combining vinegar and sodium bicarbonate (baking Soda)
You can actually make sodium Acetate at home using Baking Soda, Vinegar, Coffee Filters and a spoon. Your results may vary and we suggest caution but you can find more information on making sodium acetate here
Sodium Acetate Uses
Sodium acetate is commonly used to help increase the life of concrete through helping to seal it against water permeation. It has been used as a substitute for the epoxy to this ends.
Also commonly used with Acetic Acid to act as a buffer to maintain a consistent pH level between 4 -6.
It is also commonly used in the tanning, to increase the penetration of the dyes and tanning products. It also helps to increase the rate at which the tan is absorbed.
In the TEXTILE industry, sodium acetate is considered a dye and color intermediate, with specific use as a mordant in the dyeing process, it is also used in the in textile industries to neutralize sulfuric acid waste streams. Because of its ability to remove insoluble calcium salts, sodium acetate is further used by the textile industry to improve the wearing quality of finished fabrics.
It is also commonly used in the petrochemical industries.
If you want to make your own hot ice you will need Sodium Acetate as it is a major ingredient. You can find a formula to make hot ice below.
It is also commonly used as a polymerization catalyst and in detergents, for its thermal energy storage characteristics, You can find Sodium Acetate used in conjunction with Sodium Diacetate to increase flavors of various foods.
Is Sodium Acetate Natural?
Sodium Acetate is a salt of Acetic Acid and sodium hydroxide. Acetic acid is an organic compound, so it really depends on if the individual would consider it natural in that circumstance. Acetic acid is products naturally as fruits begin to spoil.
Quick Details:
- Synonyms: Sodium Acetate Anhydrous
- Grade: Technical Grade
- CAS: 127-09-3
- Appearance: White Powder
- Solubility: Soluble
- Natural or Synthetic: Synthetically produced from natural product.
- Recommended Retest or Shelf life: 2 Years
- Storage: Store in a cool dry, dark place. Airtight is recommended
What type of Sodium Acetate is this
We carry Sodium Acetate Anhydrous, a technical grade.
How do I know this is Good
Our Sodium Acetate is manufactured to the highest standards by one of the largest manufacturers in the world. Headquartered right here in the USA. They have been in operation for almost 100 years. We also have a 45 day, return period for our products including our Sodium Acetate.
How to Use Sodium Acetate for Dry Ice
What you need to do is saturate the water with sodium acetate at a high temperature. There are 2 ways of getting this.
Heat water to about 60 deg C, then add sodium acetate until it will no longer dissolve. Pour the liquid into a vessel, making sure you DO NOT transfer any solid into the vessel. Stick the solution into the fridge until it is cooled to fridge temperature or even room temperature. At this point, the solution is supercooled, and any trigger can induce crystallization.
Make a saturated solution of sodium acetate at room temperature (i.e. add acetate until you are left with an undissolved layer). Heat the solution to 60 deg C, which causes the remainder to dissolve. Once the solution is completely clear, pour it into a vessel and stick it in the fridge, as in method 1.
Method 1 will give you more crystals when touched, but any solid transferred will ruin the experiment. Method 2 will always work but generates fewer crystals.
There are no other specifics. You need to make a supersaturated solution, and can not transfer any solid material from the saturate.
How to Make Hot Ice With Sodium Acetate
Things You'll Need:
- Sodium acetate
- Small sauce pan
- Water
Fill a saucepan will water and heat it until it is near-boiling. Boiling is too hot, but a small amount of simmering is okay. The main thing is to make the water hot.
Dissolve as much sodium acetate into the water as possible. The more you dissolve into the water, the denser the crystals will be when they form the hot ice. Keep stirring in as much as possible until you can no longer get the sodium acetate to dissolve in the water. It is important to stir the mixture constantly at this stage.
Know that when the mixture is completely dissolved, pour it into a glass or other container. Make sure to only pour the water with dissolved sodium acetate into the glass. If any sodium acetate remains undissolved, leave it in the bottom of the pan as you pour.
Place the glass in your refrigerator to allow the water to cool.
Be aware that after the mixture has cooled, pour some into a tray, mold or whatever container you wish to hold the hot ice. As you pour it into the tray, note that it remains in liquid form.
Just touching the solution will not instigate the reaction, you must introduce a "seed" crystal. To do this, lightly moisten your fingertip so a couple of dry/un-dissolved crystals will stick there, now you're ready to touch the solution. Touch the top of the liquid with the tip of your finger. You do not need to dip your finger or hold it on the liquid. Just a quick tap is all that is needed. Hot ice crystals will instantly form where you touch and will spread through the mixture until every drop of liquid is in a frozen but hot crystalline state. In just one second you will have hot ice.
Customer reviews
Recommendation for purchase











